Adsorption processes, which also include their reversal (desorption), occur through the physical or chemical binding of material particles to the surface of the grains of the aquifer. While chemical binding to the matrix is usually irreversible, physical adsorption is generally reversible, i.e. depending on the ratio between adsorption and transport speed, an equilibrium is established between adsorption and desorption. Such equilibria can be described for constant temperature conditions by so-called adsorption isotherms with both linear (Henry isotherm) and non-linear character (Freundlich and Langmuir isotherms) taken into account in the mass transport calculation.
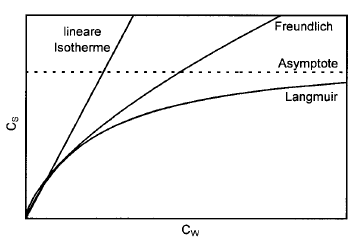
Comparison of the different adsorption isotherms
The adsorption rate is described by a function cs = f(cw):
with:
cs = concentration of the substances adsorbed in the matrix
c (or cw ) = concentration of the substances dissolved in the fluid.
All three approaches (Henry, Freundlich and Langmuir) assume a constant fluid density:
ρ0 = ρ(c=0)
 Linear adsorption according to Henry
Linear adsorption according to Henry
