For a saturated aquifer with constant density and steady state flow, the total mass flow j for mass transport can be mathematically formulated as the sum of the individual mass flows from advection (jadv), diffusion (jdiff) and dispersion (jdisp) as follows:
j = jadv + jdiff + jdisp
As the transport of the constituent only takes place in the flow-effective pore space (n) of the aquifer, this must also be introduced in a volume-based analysis.
jv = nj
with:
jv = volume-related total mass flow [(m kg)/(s kg)]
n = flow-effective pore space [-]
j = total mass flow [(m kg)/(s kg)]
The equation is obtained by substituting the individual components:
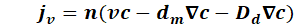
The proportion of molecular diffusion and hydromechanical dispersion can be summarised as hydrodynamic dispersion:
D = dmI + Dd
With the unit matrix I:
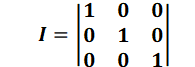
This results in the equation for the steady state mass flow:
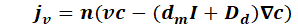
The mass balance makes it possible to observe the change in concentration over time (transient mass transport) in relation to the rates of substance flowing into or out of a control volume, taking into account sources and sinks (σ), i.e. points at which substance is added to or removed from the system.
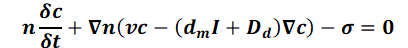
The term σ represents all mass in- and outputs, and can be broken down as follows:
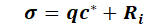
with:
qc* = volume-related addition or removal of water with the concentration c*
Ri = proportions of all non-conservative mass transport processes such as adsorption, chemical or biological degradation reactions
Using the product rule for the advection term:
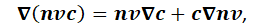
the continuity condition 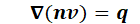
and the summary D = dmI + Dd
gives the transient mass transport equation for ideal tracers at constant density of the aquifer:
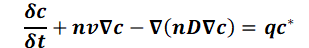
Example:
q corresponds, for example, to the attribute KNOT node removal/addition or a mass flow rate, unit [m³/TU] and c* corresponds to the corresponding attribute KONZ.
If saturated/unsaturated conditions are present in the aquifer, the saturation Sr must be taken into account in the mass transport equation:
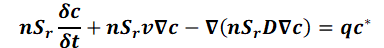
Depending on the unit of the concentration boundary condition, the unit of this equation is [kg substance / (kg solution *s)] or [kg substance / (m³ solution *s)].
 Treatment on non-conservative processes
Treatment on non-conservative processes
